Research
About Our Research
Research in the Scarpelli Lab can be broadly categorized into three areas: 1) Development of new radiotherapy strategies, 2) Development of new medical imaging techniques, and 3) Development of theragnostic (combined imaging and therapy) techniques to improve human health. More details can be found below.
Ongoing Studies
Developing Improved Radiotherapy Strategies
Ongoing projects within our lab utilize radiotherapy for eliciting antitumor immune responses. One of the first steps in initiating a systemic anti-tumor immune response is tumor antigen recognition by immune cells. This antigen recognition is amplified when tumor cells undergo immunogenic cell death, which can be elicited by delivering high radiation doses. Delivering relatively high radiation doses to tumor cells can be readily achieved with spatially fractioned radiotherapy, where an alternating pattern of low and high radiation doses are delivered to the tumor. In addition, unlike conventional radiotherapy, the low dose regions of spatially fractionated radiotherapy can spare tumor-infiltrating immune cells. This sparing further increases the likelihood of a successful antitumor immune response. To achieve spatially fractionated radiotherapy our lab uses multiple approaches including grids and lattice delivery.
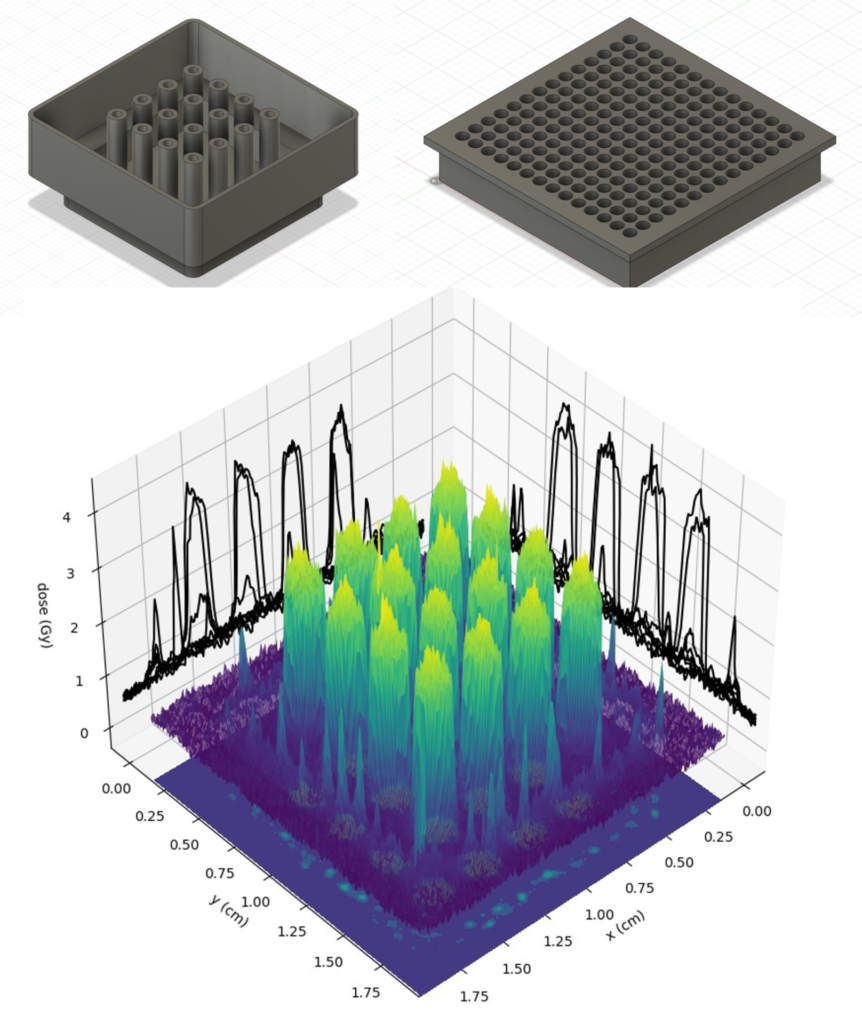
Developing Medical Imaging Techniques
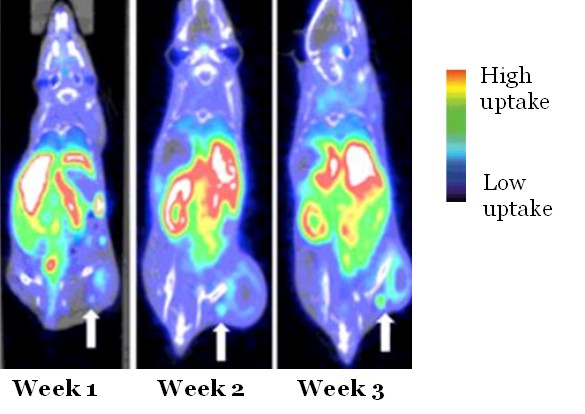
Unfortunately, there is no established clinical method for measuring immune responses within and around tumors. Due to the dynamic nature of the immune system and the immune response to therapy, conventional tissue biomarkers derived at a single timepoint (e.g., tumor resections, biopsies), generally do not provide reliable measures of the immune system. Therefore, Dr. Scarpelli’s laboratory prioritizes development of molecular PET and/or MR imaging techniques that are sensitive to these effects and could be used as a subjective marker of immune response. Specific ongoing projects include development of a new MRI contrast agent for assessing myeloid-derived suppressor cells within the tumor microenvironment and application of PET radiotracer for assessing activated T cells.
Developing Theragnostic (Combined Imaging and Therapy) Techniques
Radiotherapy is an indispensable part of the standard care for glioblastoma (GBM) patients; however, despite initial responses to radiotherapy, GBMs invariably recur. A proposed strategy for improving GBM radiotherapy involves combining both radiotherapy and therapy targeting tumor-associated macrophages (TAMs). Dr. Scarpelli’s lab is determining the cytotoxic mechanisms and anti-tumor efficacy of integrating TAM targeting within clinically relevant radiotherapy regimens. The research repurposes an established glioma imaging agent, for theragnostic TAM targeting. Given the prevalence of radiotherapy for treating cancer, development of this therapeutic approach has the potential to improve outcomes for many patients.
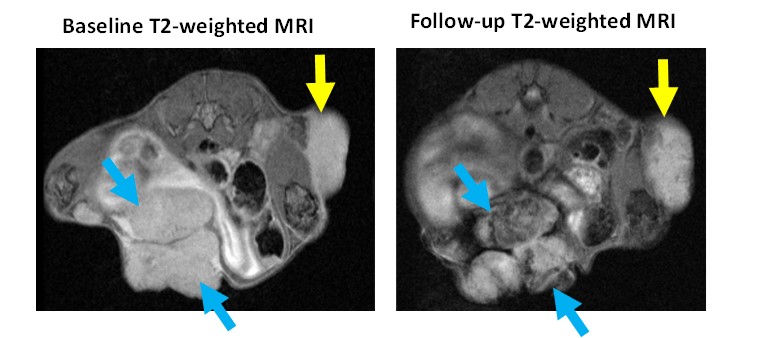
Dr. Scarpelli’s lab also evaluates metal nanoparticles as radiosensitizers for tumor cells. The focus has been on utilization of iron-oxide nanoparticles as these can be assessed using MRI and also lead to radiosensitizing effects, thus enabling a theragnostic approach. Dr. Scarpelli’s lab is currently evaluating the radiosensitizing potential of multiple iron oxide nanoparticles. This includes a HER2 targeted nanoparticles for HER2 positive breast cancer and an untargeted iron nanoparticle for other cancers.
